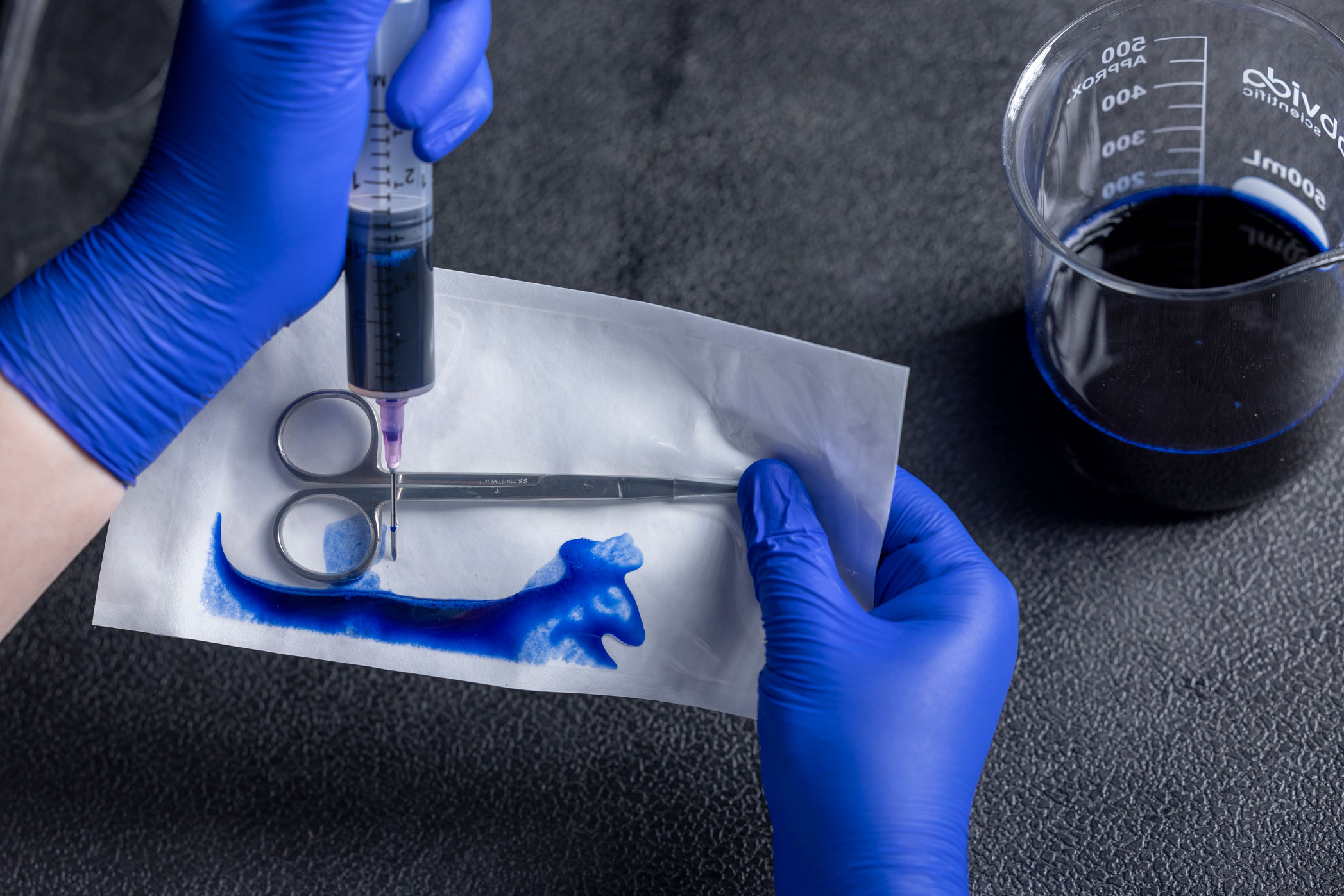
Dye Leak Test
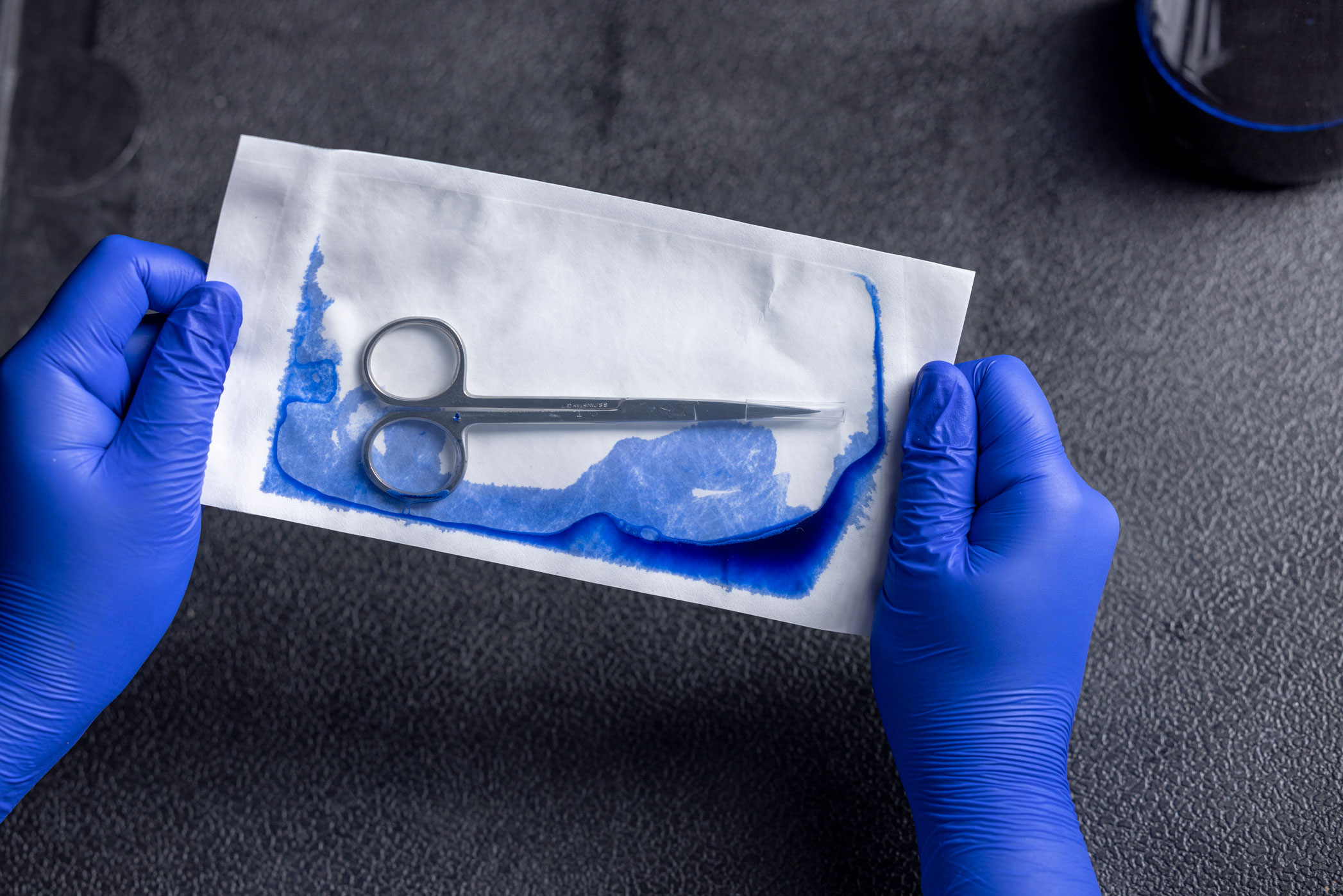
The ASTM F1929 is intended to identify seal integrity defects, such as channels, voids, incomplete seals, or other defects of the sterile barrier. The test is most often used when the sealed material includes transparent film on one side and a porous sheet material. This is accomplished by injecting a toluidine-blue dye solution into the sealed package and allowing it to pool along the seal for up to 5 seconds. Any channels, voids, or other breaches of the sterile barrier will turn blue as the dye seeps into the space and stains it, making seal defects easy to see. Other variations in technique are offered within the standard.
Industry Application
The dye leak test evaluates seal integrity. It is typically performed during sealer validation or for in-process maintenance. The sensitivity of this test is claimed to be 50 microns. The test is performed using a specialty dye solution that has very low surface tension, meaning it will seep into smaller crevices than ordinary water is capable.
NOTE: This test is not intended to be a whole-package integrity test. See Bubble Leak Test.
Frequently asked questions
What is dye leak testing?
Dye leak testing per ASTM F1929 is a test method that is used to identify leaks caused by channels between a transparent film and porous sheet material. Dye leak testing per ASTM 3039 is a test method that identifies leaks between transparent film to film packaging.
How sensitive is Dye leak test per ASTM F1929 and ASTM F3039?
Dye leak testing per ASTM F1929 and ASTM F3039 can detect breaches in the sterile barrier down to 50 microns.
