
Bubble Leak Testing
Bubble leak testing per ASTM F2096 can be used for non-permeable and most permeable materials such as paper and Tyvek. The test method is destructive and is intended to determine sterile barrier integrity of an otherwise sealed package.
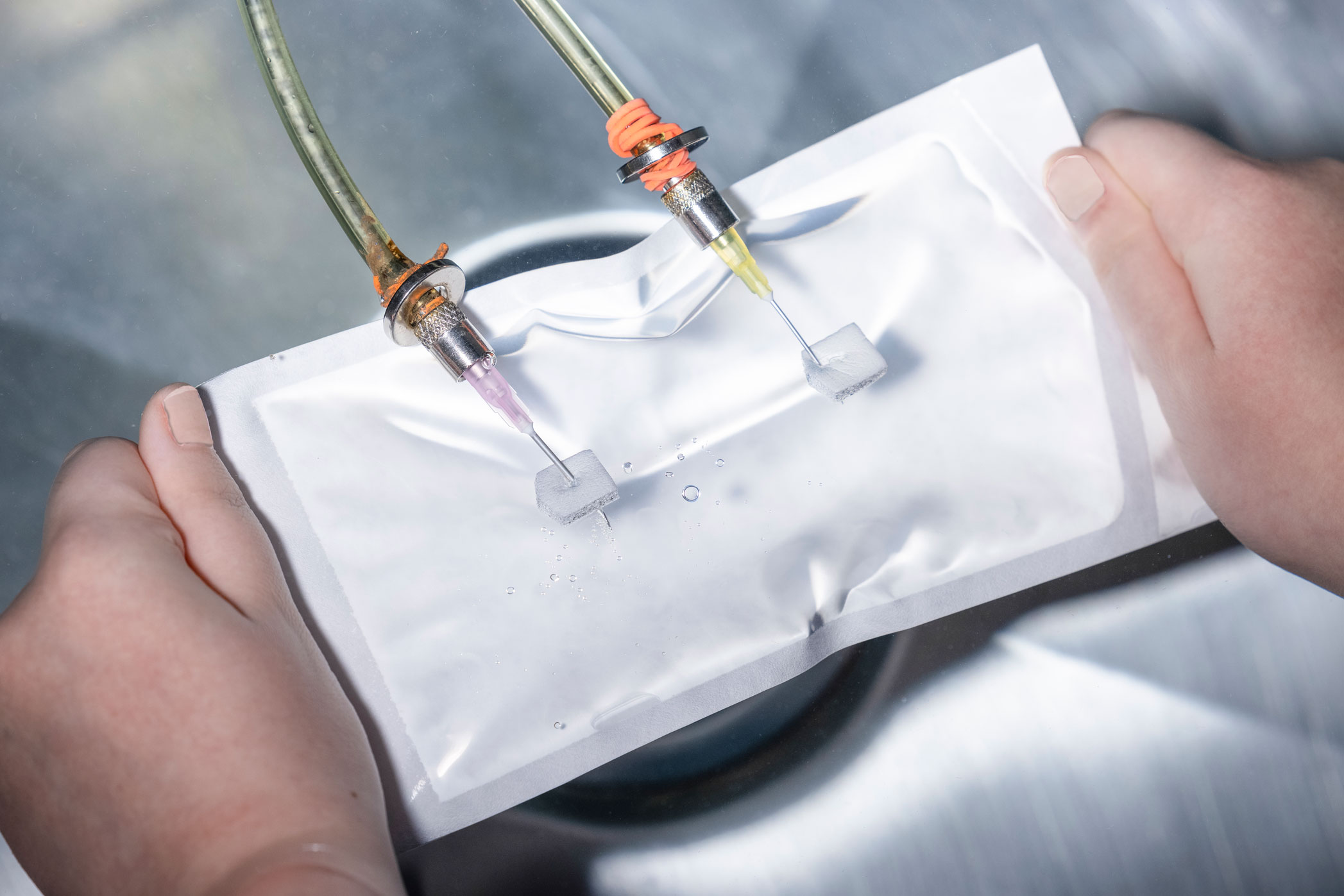
Industry Application
Bubble leak testing is a whole-package integrity test. It is typically performed as part of a validation activity, such as a sealer validation, transit validation, or shelf life validation. ASTM F2096 bubble leak testing has a claimed sensitivity of approximately 250 microns. It is performed by submerging the package in water, inflating the package with air while it is underwater, and observing for a steady stream of bubbles emanating from the package, indicating a breach of the sterile barrier.
Frequently asked questions
How sensitive is bubble leak testing?
The bubble leak test method per ASTM F2096 can detect a breach in the sterile barrier system down to 250 microns.
How is bubble leak testing performed?
Bubble leak testing is performed by submerging a package into water and forcing air into the package. If a steady stream of bubbles is observed, that would indicate a breach of the sterile barrier.
