
Ensuring the integrity of the sterile barrier is paramount when validating medical device packaging. ASTM F2096 (Bubble Leak Testing) and ASTM F1929/F3039 (Dye Leak Testing), are two of the most common test methods performed in the medical packaging industry. Is it necessary to complete both tests when validating your packaging? This is a question we hear often. To answer, we’ll first explore these tests and their significance in more detail.
What is Dye Leak Testing?
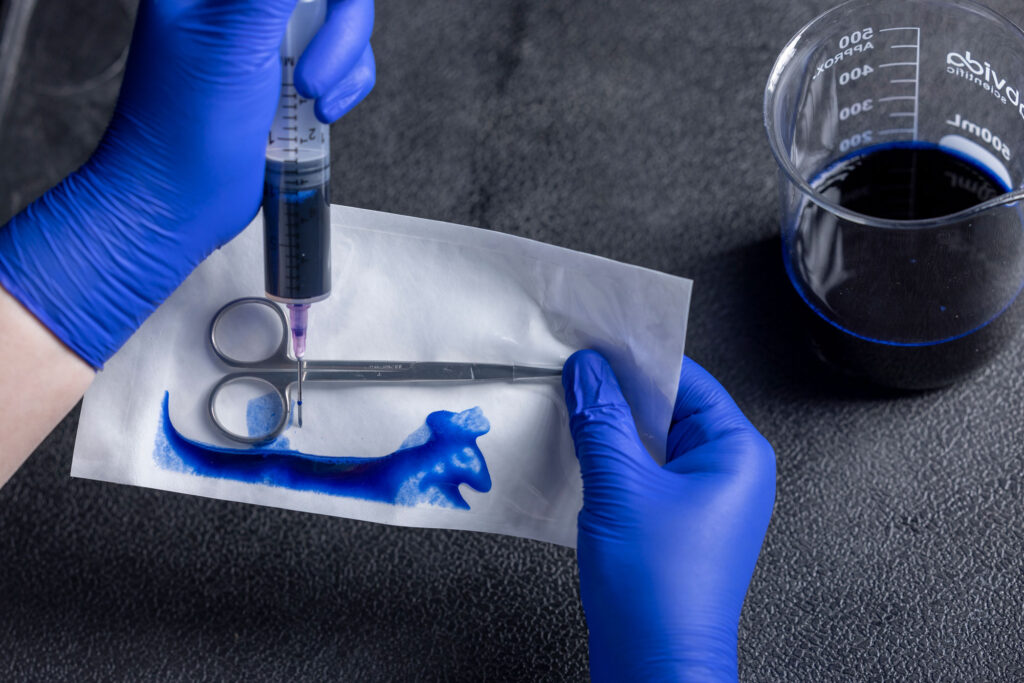
The two prominent standards for the dye leak test are ASTM F1929 (Porous) and F3039 (Non-Porous). Dye Leak Testing focuses specifically on evaluating the seal integrity of medical packaging. A blue dye is injected into the packaging and held in place for 5 seconds to identify any seal defects, such as channels, voids, incomplete seals, or other imperfections within the sterile barrier.
Dye leak testing is a crucial quality control measure in the medical packaging industry. Defective packaging could compromise the sterile barrier and lead to contamination of the medical device or product contained within.
The ASTM F1929 standard primarily uses porous materials for packaging, such as Tyvek.
On the other hand, ASTM F3039 is applied to non-porous packaging materials, such as foil and film packaging.
The 2023 revision of ASTM F1929 and ASTM F3039 removed Triton X-100 as an ingredient required to make the blue dye because the EU banned it. Learn more about the research that went into potential Triton-X 100 alternatives.
What is Bubble Leak Testing (ASTM F2096)?
Bubble Leak Testing serves as a whole package integrity test for medical packaging. Typically integrated into validation activities for transit and shelf-life assessments, this test method is performed by submerging the package underwater and inflating it with air to a pre-established pressure. Technicians look for steady streams of bubbles emanating from the package, indicating potential breaches in the sterile barrier system.
Bubble Leak Testing is essential for complying to ISO 11607 for your sterile packaging validation.
Dye Leak Testing vs. Bubble Leak Testing: The Difference and Integration
While both ASTM F1929/F3039 and ASTM F2096 fall under the umbrella of integrity tests, they serve different purposes. Bubble Leak Testing assesses the integrity of the entire SBS, focusing on sterile breaches in the whole package. This makes bubble leak testing an excellent package integrity test during performance and stability validations. Dye Leak Testing only tests the seal integrity. In the Operational Qualification (OQ) and Performance Qualification (PQ) of your process validation, Dye Leak Testing is suitable.
By understanding the roles of each test and by leveraging expert advice, you can navigate your packaging validation with confidence. If you have other questions regarding your packaging validations, contact us today to meet with one of our packaging professionals!
Both ASTM standards undergo regular revisions, and PCL’s Director of Packaging Innovation, Matthew Terbush, contributes to ASTM committee that review and publish these standards. With PCL’s expertise in both bubble leak and dye leak testing, rest assured that you’re in capable hands for your packaging validation needs.