

How to determine the best temperature for a product’s accelerated aging testing using a standard like ASTM F1980 is a common question from clients. It doesn’t always have a right or wrong answer. The benefits of aging your product at a higher temperature is that it simulates the aging interval faster, but it has risks to different products. Everyone wants to age their products at the highest possible temperature that they can, to pull the lead times in as much as possible, but there are risks and trade-offs to that. We have had clients age their products at two separate temperatures for the same aging interval, that way they are able to pull their timeline in if the higher temperature products are able to pass the integrity testing.
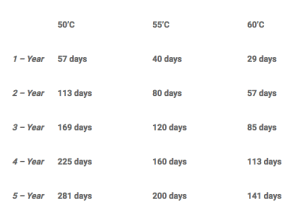
Let’s use 50’C and 60’C as an example, following ASTM F1980 Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices aging products to a 1-year interval at 50’C takes roughly 57 days whereas 60’C will only take 29 days. The most common temperatures seen at PCL are 50’C, 55’C, and 60’C which it is no coincidence that we dedicate our largest Walk-In chambers to those temperatures. We have aged products as high as 70’C and as low as 45’C and we do have space at PCL to be a little more custom to certain product lines. Below is a table of 50’C/55’C/60’C and different breakpoints for yearly intervals. This is all assuming a real-time storage temperature of 23’C and Q10 = 2.
A good start to picking the appropriate temperature is to find out if your product/device will be at all affected by long durations of high heat and low humidity. This also applies to your packaging materials. These types of questions should all be addressed in the FMEA (Failure Mode Effects Analysis) document. If any of the three (product, device, or packaging materials) cannot handle high heat and low humidity for a long duration you may be forced to age your products at a lower temperature that will take longer to simulate the yearly interval you are striving to hit. These are all considerations to take as you move into shelf life claims for your device and its packaging.