
USP <671> was recently revised to include an alternative test method for determining moister vapor transmission rate (MVTR) using water rather than the desiccants. In this blog, we’ll explain the two test methods and the benefits of the new test method.
What is MVTR?
An MVTR test, sometimes referred to as water vapor transmission rate (WVTR) test, is a test procedure that does exactly what its name says. MVTR is a way to measure the passage of water vapor through a material. From a pharmaceutical package (USP <671>) testing perspective, it measures how permeable a single unit and/or unit-dose blister card barrier is.
Depending on what the class of the packaging is supposed to determine how much moisture is “allowed” to transfer through the packaging. The amount of moisture transfer we are talking about here is often just a few water molecules. This means a special kind of scale must be used so that it can weigh the .00001-gram fluctuation.
Existing MVTR Test Method – Desiccant
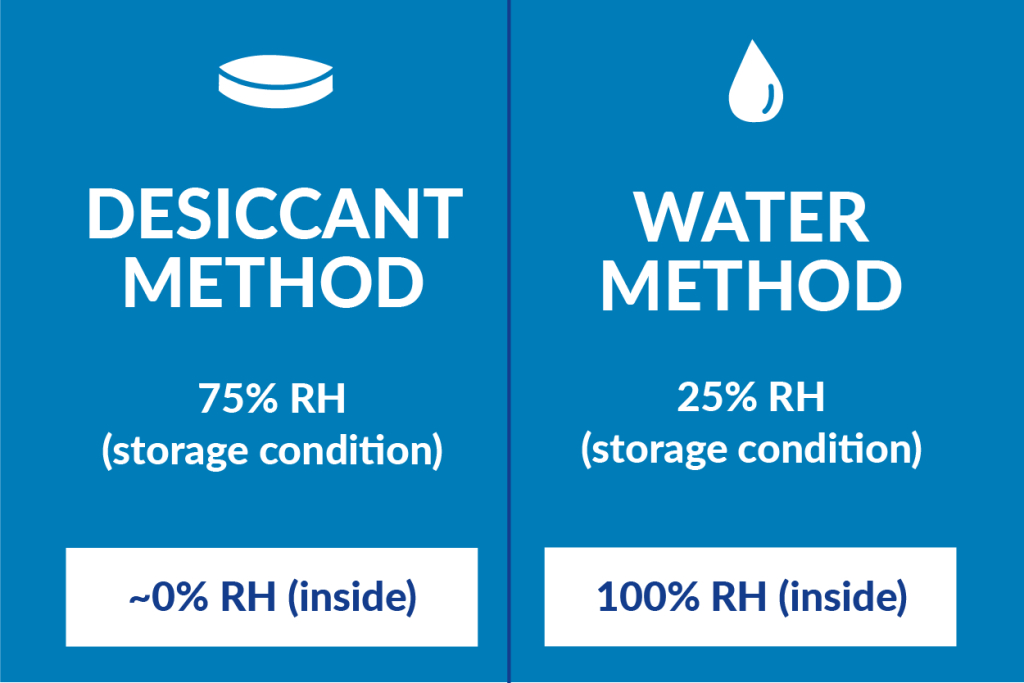
Desiccants are water absorbers; therefore, this method tracks weight gain to determine the class of packaging. The interior RH for this method is ~0% which means the RH in the chamber (the storage condition) would need to be 75%.
There are 5 durations of time that this test can run for (7-days, 14-days, 21-days, 28-days, and 35 days). Depending on what length your study is running from determines the frequency at which the samples must be removed and weighed. Once the samples are weighed, they are placed back into the chamber until the samples are due to be weighed again.
When the study is complete, the weight gain is attributed to a class the packaging receives.
Potential Discrepancies With The Existing Desiccant Method
- If there are variances in the size of desiccants, or if desiccants are missing from some blisters, then there could be errors in the findings of the study.
- When desiccants are not dried completely before being added to blister packs this means the interior of the blister is not 0% RH and could get up to 10% RH.
– If there is already moisture in the desiccant that means not as much water can be absorbed, which could lead to a discrepancy in the study without the lab techs being aware of it.
MVTR Water Test Method
The water method is performed the same way but instead of using desiccant, it uses water. With this method, the interior RH is always 100%. Since the interior RH would always be 100, the RH in the chamber would be a low 25%. The main difference for this test method is that weight loss is calculated rather than weight gain then that difference gives you the class rank for your packaging.
Benefits of The Water Method
- With water, there is not a correlation between the variability of fill amount and internal RH.
- If there are 3 drops in all the blisters but 1 of the blisters loses a drop, that will not affect the results of the study.
- The stability of the internal RH due to the use of water allows holding the samples or reusing them later for a different study if there is still water in all the blisters.
- This means additional measurements or studies are possible without concern over depleting the water. This is not feasible with the desiccant method.
- The uncertainty of having adequate desiccant which will maintain an inside RH near 0% is eliminated by creating a constant inside RH of 100% with water.

The primary reason that the water method is more precise is that you can be confident that there is no variance of the internal RH whereas there is always a chance that there will be variance with the desiccant method.
Contact us today if you require MVTR and would like to set up a conversation with one of our medical device packaging engineers!
*Note: The benefits of using the water method we’re not as significant for multiple-unit container closure systems (ie: a bottle of capsules) as for single-unit blister container closure systems (blister pack).