Has your company dealt with unique device labeling? As the FDA digs deeper into risks associated with sub-performing packaging labeling systems, UDI is emerging as a packaging industry hot button.
Understanding UDI Labeling
A complete UDI label contains two parts:
- “DI” – Device Identifier, such as a Global Trade Identification Number (GTIN)
- “PI” – Production Identifiers, such as a lot code or expiration date
The complete UDI is printed onto each package label as both a barcode and human-readable text.
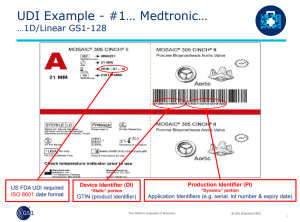
Example UDI Label – Credit to GS1, accessed here
More About Device Identifier
In order for companies to grow and expand, they must have the ability to communicate to new markets around the globe. This is where the Global Trade Identification Number comes in. GTIN is the global language that allows companies to effectively identify, capture, and share information automatically and accurately no matter who and where they are. GTINs can be acquired through accredited issuing agencies such as GS1.
How does it work? First GS1 assigns a Company Prefix. This prefix will allow businesses around the world to identify that company. Next, using GS1’s online portal, unique GTINs are obtained for all products under your scope. Defining your scope and understanding what requires a GTIN vs. what doesn’t is of critical importance. PCL specializes in helping companies with UDI implementation and can be a great resource to walk you through the considerations and ensure successful scope definition.
More About Production Identifier
This take can take many forms – essentially the Production Identifier “PI” is anything that creates a unique fingerprint for the production of the device, such as a lot number, expiration date, manufacture date, etc. Good news is that medical device manufacturers are already doing this, so now it’s a matter of simply plugging that information into the UDI format. GS1 accomplishes this by categorizing information into “application identifiers” (“AI”) which you can select from. For example, expiration date is coded by the number (17) in parenthesis. When the barcode shows (17) followed by [DATE] this means “expiration date”.

Example showing (17) Expiration Date Coding
Barcode Verification
Once the barcode is created, verification and validation is the best way to ensure barcodes scan properly every time. Verifying the content and quality of your labels saves time, productivity and money in the long run. PCL offers barcode grading as a service which assesses GS1 general specification codes and verifies for print quality, quit zones and other criteria.
The Big Picture of UDI
UDI at its core is simple and powerful – GTINs combined with production identification numbers such as lot numbers, expiration dates, manufacturer dates, etc. uniquely identify your company and product around the world. The use of barcodes allows labels to be scanned so the information stored can be easily and automatically accessed. Using scannable barcodes to access information increases the efficiency, productivity and customer satisfaction across the supply chain. Inventory management and traceability, point of sale accuracy, regulatory compliance, shipping and receiving, also improve.
Worried about UDI? Feel free to ask PCL your question. Our live chat allows you to ask an engineer, or contact us for a bigger conversation. We routinely help companies set up, implement, and validate UDI compliant barcodes through both project management and testing.