

Standards. Requirements. Regulations.
Medical device manufacturers address thousands of details to ensure their products get to market efficiently and safely, all focused on providing patients with excellent products and materials that help them recover, heal, and enjoy life.
The medical device packaging industry also meets rigorous standards that are constantly reviewed for efficiency and scientific validity. Packaging Compliance Labs’ team of experienced packaging engineers actively participate in industry organizations where their knowledge and expertise lead to better standards and business practices.
In 2022, the standards for the ASTM D4169 for transit testing are changing.
“My responsibilities are to make sure the lab is adhering to all these new standard revisions so that our clients have confidence their packages meet FDA testing requirements,” says Matt TerBush, Senior Laboratory Packaging Engineer.
What is ASTM?
The American Society for Testing and Materials (ASTM) is an internationally recognized body that establishes more than 12,500 global standards that positively impact public health and safety, consumer confidence, and overall quality of life. Standards relate to test method, specification, classification, practice, guide, and terminology standards.
ASTM was founded in 1898 and includes more than 32,000 member volunteers across more than 140 countries. There are more than 150 technical committees from industry and consumers that develop and maintain ASTM standards that provide requirements for quality, safety, and performance.
Our team of packaging specialists contribute to robust conversations and evaluations of standards that protect the safety and well-being of consumers across the globe.
What Changed with ASTM D4169 Transit Test?
ASTM D4169 is a common standard for distribution simulation testing that includes vibration/shock, drop testing, high altitude, etc. ASTM D4169 simulates an extreme shipping environment to appropriately challenge many types of packaging systems. The standard was last updated in 2016 and was due for its standard five-year review.
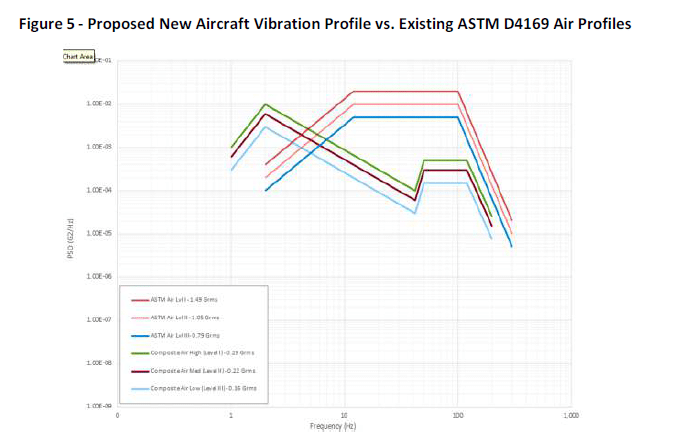
In recent verification activities and analysis, engineers discovered that the air profile differed significantly in shape and intensities that what was previously deemed acceptable. In fact, the air profile was last updated more than 40 years ago when the testing process was challenging to measure and verify. In fact, during the original testing, there was an issue with an international shipment that got held up in customs, and the testing process had to be abandoned because the item couldn’t last during the delay. More recently the committee executed a variety of new timed studies and concluded that the vibration levels from wheels up to wheels down differed from the existing standard required.
This was a big a-ha moment for ASTM committee members, who recommended that the requirements be changed. The new testing process will now include “low,” “medium,” and “high” segments of the profile to account for different levels of intensities as observed during the new data collection and analysis. As a result of the revised requirements, packages will be exposed to a better representation of what is happening in real-world situations.
What Does This Mean for Medical Device Manufacturers?
Peace of mind and confidence that your packaging testing providers are on track to meet ASTM standards and FDA requirements. Your package testing validation processes will continue to measure and conform to the latest standards for air vibration intensities.
PCL recently expanded our transit testing equipment to meet small and large medical device manufacturer schedules and budgets to launch new products to the market; our processes meet the new ASTM D4169 transit testing standards.
“Not only are we expanding our capabilities and services for clients,” says Matt, “but we’re also subject matter experts who stay up to date with current revisions and methods and stay involved in these important decisions on behalf of our clients.”
Rest assured that PCL keeps up with the standards, and is an active, authoritative voice in the testing processes and verification requirements for medical device packaging.