

To ensure a sterile barrier system is effective, it must go through a variety of tests including aging. Aging studies are performed to verify that the sterile packaging and contents will remain intact for a medical device’s fully intended shelf-life (ex. 5 years). There are two kinds of aging tests: accelerated and real-time. With the help of Alec Respecki, a packaging engineer here at PCL, we’ll break down the details of aging and the pros and cons of the different testing options.
Real-Time Aging
The FDA requires real-time aging results. Per ISO 11607 Section 8.3, a consensus standard formally recognized by the FDA, the product-packaging system must be aged for the full duration of the anticipated shelf-life at the intended storage conditions. In order to expedite industry innovations, the FDA allows for accelerated aging to speed medical devices to market. However, real-time aging must be performed in tandem so that the accelerated aging results can be verified.
Accelerated Aging
Accelerated aging is exactly as it sounds; aging testing dialed up a notch or two. The device is packaged and placed in a specialized chamber that can simulate a variety of temperatures. 40 days in the chamber at 55 degrees Celsius (131 degrees Fahrenheit) is equivalent to 1 year at 23 degrees Celsius (73.4 degrees Fahrenheit). The length of time in the chamber is directly related to the shelf-life of the device. The higher the temperature, the less time your sterile barrier system must stay in the chamber.
The chart below provides a general idea of the relation between time and aging temperature:
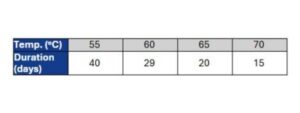
Check out PCL’s accelerated aging calculator to see how long your specific project might take!
This test method shortens overall timelines and allows device manufacturers to launch their product with preliminary aging data. However, it’s imperative that you proceed with caution when raising the temperature for your accelerated aging testing.
Dual Temperature
Oftentimes, medical device manufacturers (MDMs) are facing truncated timeframes and possibly a lack of funding. So, increasing the temperature and shortening their accelerated aging timeline can look like an appealing option to an MDM who is eager to get their device to market. However, there are risks associated with this testing choice. It can cause failures that aren’t representative of actual aging results. If your samples fail during testing, you must start over with new packaging samples, causing significant and costly delays.
If you are interested in increasing the temperature for your aging test, you can run parallel studies, one at an increased temperature, and one at the standard 55C. There are a few pros and cons to this.
Pros:
- Time Savings
- If your aging samples pass integrity testing at 65C, you could shave valuable time off of your project timeline
- Less Risk
-
- Running dual temperature testing allows for added flexibility within your shelf-life validation.
-
- If failures are observed at the 65C level, you can leverage the data from the 55C study
-
- Limited delays since the 55C study is running in tandem with the higher temperature testing.
Cons:
- Increased Sample Size
-
- A separate set of samples is required for each aging temperature in order to meet sample size requirements
-
- Example: each aging interval/temperature requires 90 samples when running at 95% confidence 95% reliability
-
- Many manufacturers experience device and material constraints

Sheet Separation
- Many manufacturers experience device and material constraints
-
- Added expense of laboratory testing
- Non-Representative Failures
-
- Packaging materials can degrade above 60C
-
- Adhesive used to form package seals can weaken = Seal Creep + Integrity Failures
-
- Film Delamination, Sheet Separation/Tearing of Tyvek = Aseptic Presentation Failures
-
- Failures do not reflect real-world conditions (won’t experience the high temperatures of accelerated aging)
- Project Delays
-
- Failure(s) will require the re-execution of the aging study
-
- Added cost of sample creation, sterilization, and lab fees
-
- Time delays from reprocessing test samples
If you’re looking to mitigate risk and receive early results, dual temperature accelerated aging testing is a viable option Remember, no matter the path taken for your accelerated aging study, you must also run a real-time aging test in tandem to be FDA compliant.
Key Takeaways
Aging tests will determine how well your packaging system performs over the full shelf-life of your device. It’s an important test that determines if the sterile barrier will remain intact until point of use. Real-time aging is required by the FDA, but accelerated aging data can be used to speed a device to market, as long as real-time aging testing is in-process. There are many pros and cons to the different aging temperatures you can choose. For accelerated aging studies, erring on the side of caution is beneficial to your overall project timeline. Sticking with the standard 55C or utilizing dual temperature testing can help save you time and money.
