

When it comes to deciding on device containment, there are two roads medical device manufacturers can go down: backer cards or thermoform trays. For this week’s blog post, we had the opportunity to work with one of our trusted industry partners, Beacon Converters, to discuss everything die-cut backer cards. Read along to learn how die-cut backer cards make a cost-effective and sustainable alternative to thermoform trays!
Benefits of Using a Backer Card
Quicker turnaround time for design. Typically, it takes less than half the time to get a layout completed for a backer card than it would take to get a design for a thermoformed tray. It is not unusual to complete the layout and design phase within days.
Great for containment. Backers have the capacity to be customized with tabs and other features that hold the device to the backer. If there are sharp features to the device, the backer can be die-cut to fold over the sharp feature of the device and “blanket” it from puncturing the pouch. Backer cards have the unique ability to provide 360-degree wrap-around protection that is not possible in a thermoformed tray.
Backers are compatible with various methods of sterilization. Backers can withstand ETO, Gamma, and E-Beam which covers most of the sterilization methods.
Design Inputs for a Backer Card
As mentioned, the main reason a backer card would be the preferred material for a medical device is if the device and all its components need to be locked in place. Medical devices that would work well in a sterile package system using backer cards are coil products, tubing, catheters, and devices containing sharp features that could puncture a pouch.
What is the alternative to Backer Cards?
Backer cards, or die-cut insert cards, are often compared to thermoformed trays because they both have the purpose of containing a device from moving or shifting in the package. A thermoformed tray with a retainer is great for containment but it’s more expensive and would require more time to engineer a solution and then manufacture.
The Sustainability Piece
Flexible HDPE backers are more sustainable in many ways, but first, we must address why it’s imperative that sustainability is a major factor in the sterile barrier system used for medical devices.
The healthcare industry has witnessed an unprecedented growth of single-use medical devices in the last decade. More single-use devices being produced means more of a burden of discarded packaging on the healthcare waste systems.
This is a hard problem to solve but using more sustainable packaging can help minimize healthcare waste to landfills.
Thermoforming Uses More Energy to Produce than Backer Cards
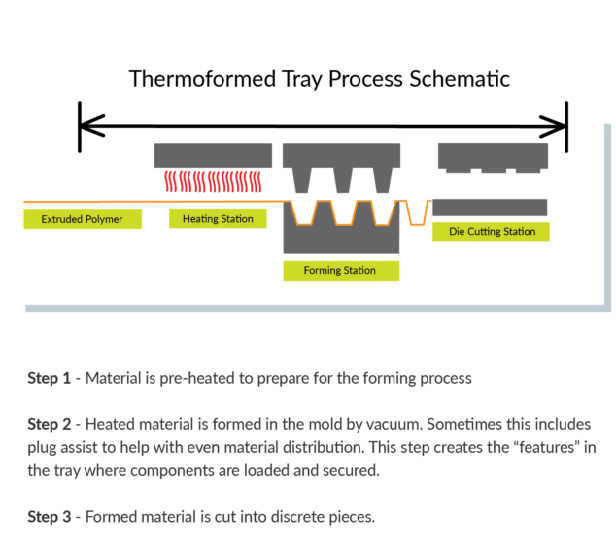
Thermoforming literally translates to ‘using heat to give shape’. For a sheet of plastic film, it must be heated evenly to a point that it can take shape and hold form. The forming process involves heat and vacuum. The formed sheet is then die cut that can provide features such as rounded corners or finger tabs.
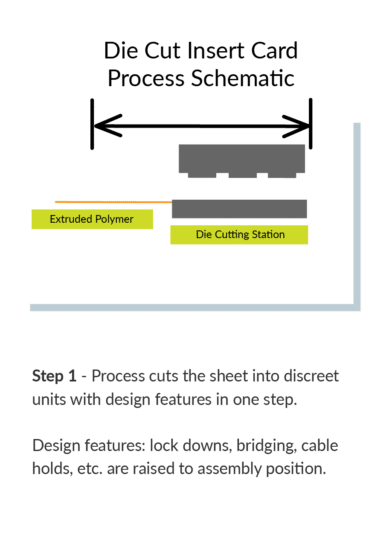
Flexible HDPE material can be formed in just 1 step.
Shipping Costs
If you are going with an HDPE Backer Card, they ship flat. Think of a stack of printer paper, this is exactly how backer cards are stacked and shipped. In one shipping box, there could be 500 backer cards in one box. This makes shipping for this material very cost-effective.
On the flip side, thermoform trays are larger and can fit a few as 30 in the same shipping box that can hold 500 backer cards.
Recycling
Backer cards are recyclable and made of HDPE; a plastic that is recycled worldwide. Cards can be collapsed, folded, or rolled to take up less room in hospital recycling receptacles. Thermoform trays are typically recyclable, too. Their size and rigid, the molded form makes packaging made with this material harder to condense.
The Amount of Material Used for Both Types of Packaging Achieves the Same Ends
If your package uses a more sustainable option will it work as well? Of course! The material used for sterile barrier packaging has no effect on its effectiveness regardless of its environmental footprint.
The key objectives of a successful medical device package are detailed in ISO 11607 1 and 2:
- Protect the device from physical damage
- Protect the device from undesirable environmental conditions
- Allow device to be terminally sterilized
- Allow the sterility to be maintained until the product is dispensed
- Allow aseptic presentation
Evolving packaging concepts consider sustainable principles and can achieve and even exceed rigid packaging formats using exclusively flexible components (backer cards). The typical challenges of size, fragility, complexity, and need for rigidity can be met with a flexible solution.
True to its principles, backer cards use less energy to produce, lower gauge material, and less volume of empty packaging which translates to less shipping volume and less ware space.