
CompliancePack™
CompliancePack, or CPack, is PCL’s ISO 11607-compliant pre-validated packaging solution for medical devices. PCL contract packaging clients can save months on their device launch when they use CPack for their sterile packaging. Our CPack portfolio consists of single and dual-barrier nylon and Tyvek pouches. We also offer a dual barrier Tray with Tyvek lids.
Who is CompliancePack™ for?
PCL’s Contract Packaging Clients
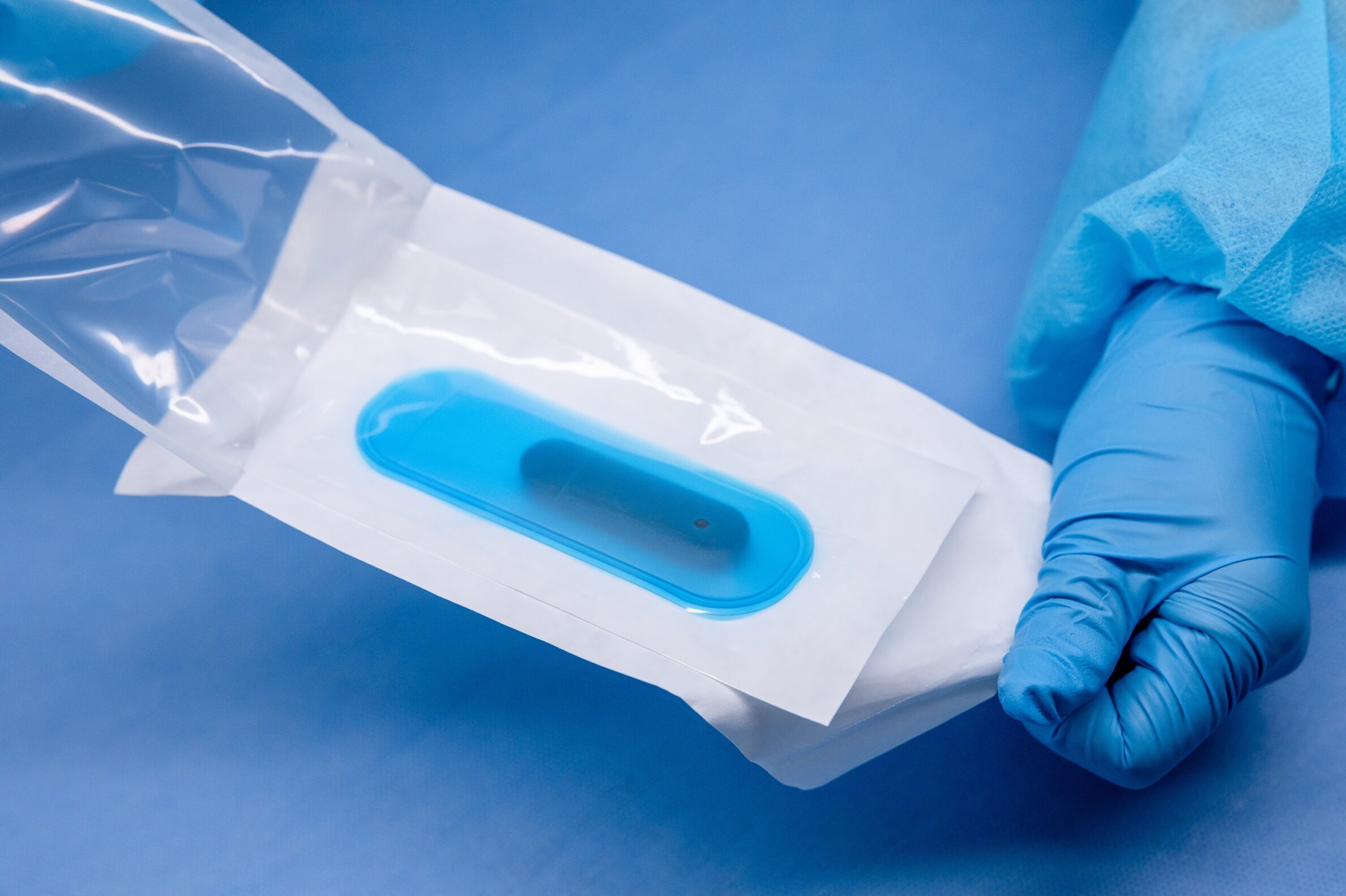
CompliancePack™ Pouch
- General purpose use
- CPack pouch materials: 100 ga BON (nylon) // 1073B Coated Tyvek
- Three dual-barrier pouch options
- Four single-barrier pouch options
- Three TPU sleeve options
- Have PCL fit check your device in standard sizes
- Customized pouch sizing is also available.
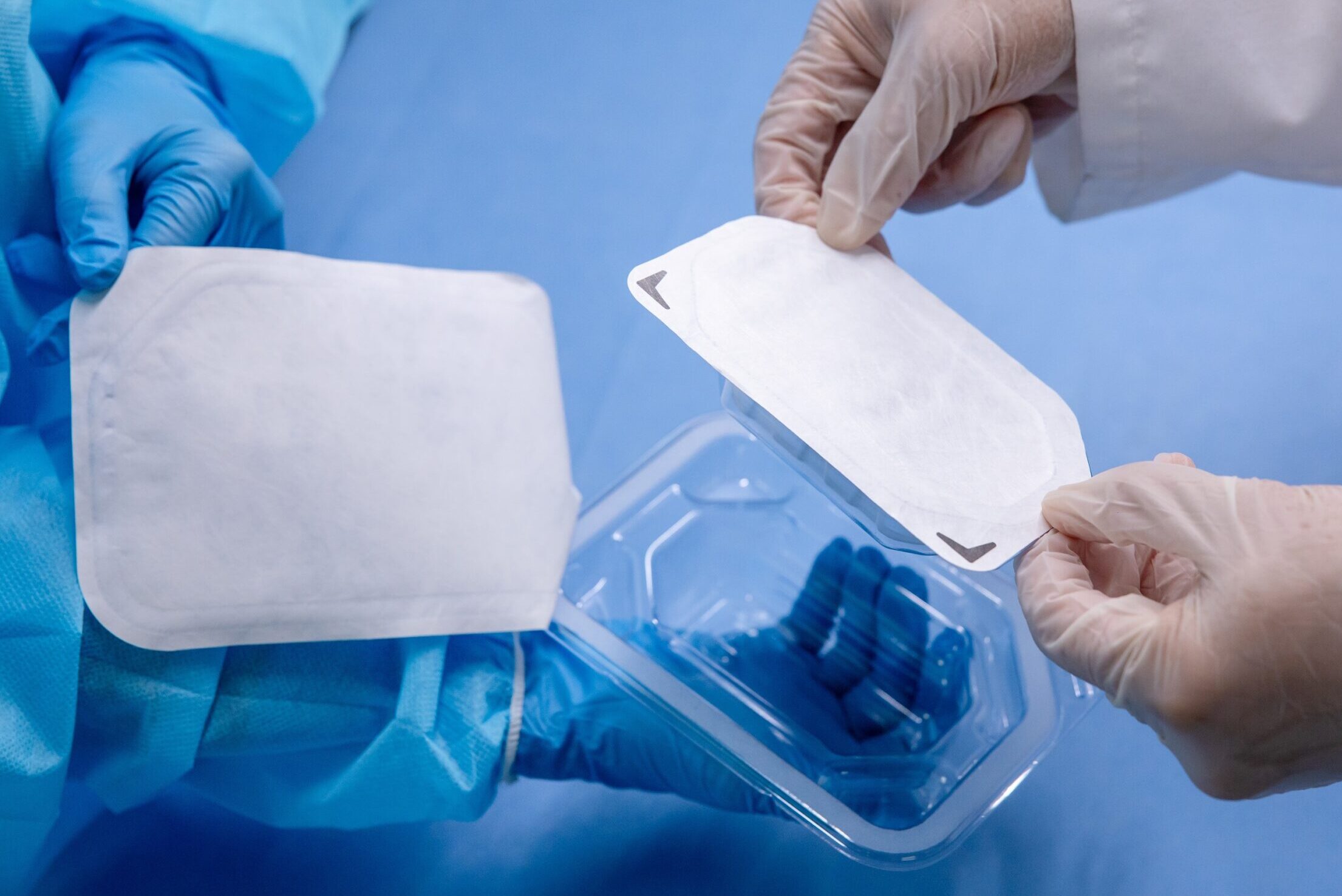
CompliancePack™ Tray
- Ideal for, but not limited to, small orthopedic devices
- CPack tray materials: PETG // ST-7385B Coated Tyvek
- One dual-barrier option
- Includes a dual barrier system option, device containment, and shelf carton
What is Included in CompliancePack™ Validations?
CPack Pouch and Tray options have been validated to the following:
- Sealing Process Characterization (DOE)
- Operational Qualification (OQ)
- Performance Qualification (PQ – Tray Only)
- 5-Year Accelerated Aging
Your packaging will still require product-specific testing, such as Process Qualification (pouch only), transit testing, and real-time aging.