
Overcome Small Batch Challenges
PCL’s ISO13485 Contract Packaging Services are made possible by our state-of-the-art ISO Class 7 cleanroom facility.
Designed specifically for low to medium-volume packaging. Because we believe patients deserve faster access to your device.
The planning, prep, and manufacturing are done.
Packaging is the final step. We've got you covered.
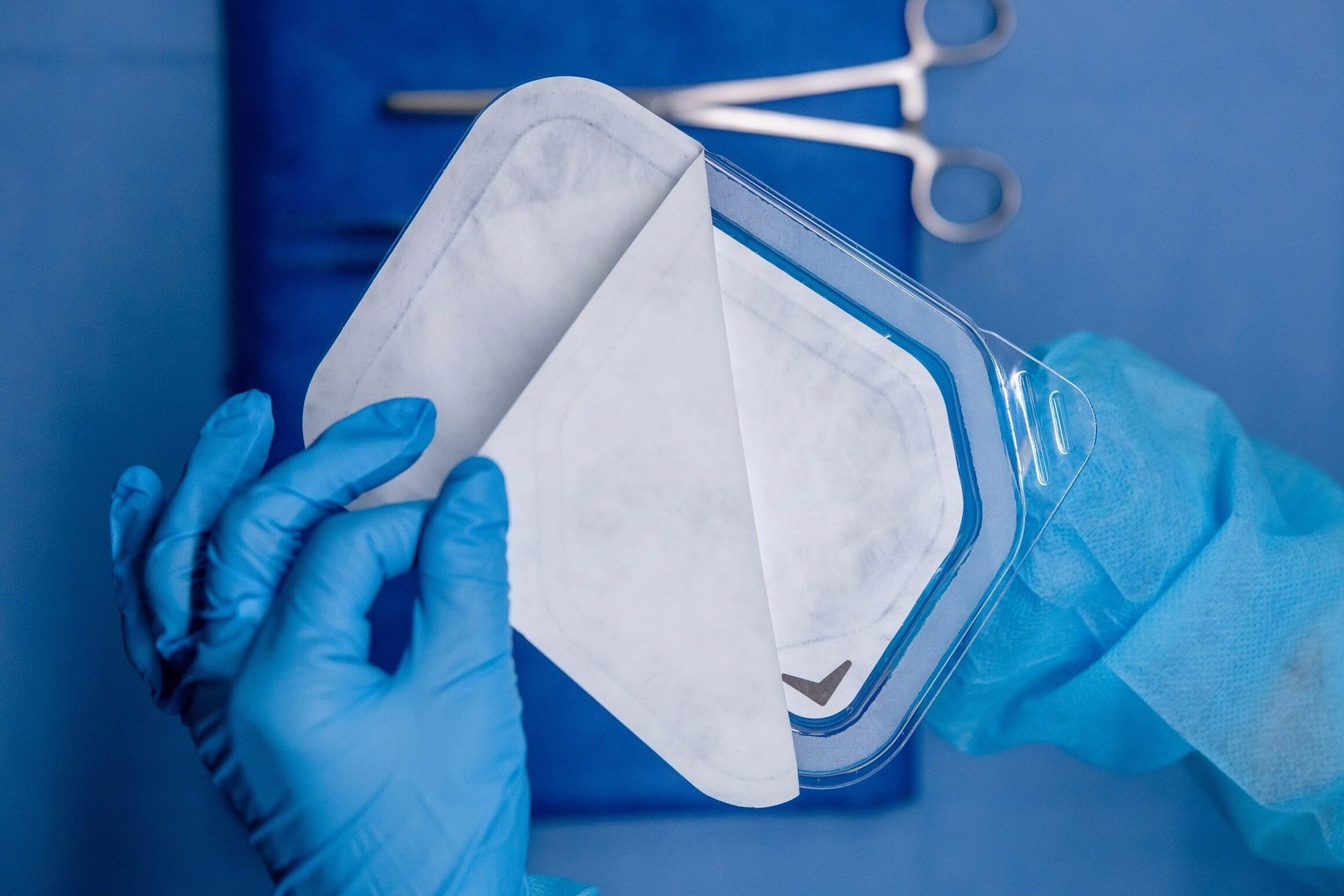
Pre-Validated Packaging
CompliancePack™ (CPack) is an expedited sterile packaging solution developed by Packaging Compliance Labs (PCL), designed specifically to meet ISO 11607 requirements. Leveraging CPack enables medical device manufacturers to significantly accelerate their device launch timelines, potentially saving months in development and validation. Available in both tray and pouch configurations, CPack offers flexibility to suit diverse packaging needs, ensuring compliance without compromising speed-to-market.

Sterile Product Contract Packaging
All medical device packaging and sealing operations are conducted within our ISO Class 7 cleanroom. Services include light assembly, labeling, and sterile packaging sealing.

Sterilization Validation & Sterile Product Management
Achieve sterilization while we manage the process for you. We partner with third-party sterilization providers to ensure the correct sterilizing agent and the characterized exposure required to achieve sterility. Methods include Gamma, EO, and EBeam.
Learn More About Sterilization Validation & Sterile Product Management

Medical Device Precision Cleaning
PCL helps determine if your medical device needs a formal cleaning process before packaging and sterilization, and offers precision cleaning solutions like parts washing and ultrasonic cleaning to support validation and routine cleaning.
We’ll oversee packaging from start to finish and get those small batches to market.
Patient Safety First
Become the Expert
Go the Extra Mile
We don't just engineer and test packaging.
We DO packaging. And we let you celebrate another satisfying launch.
Explore more PCL Services

