

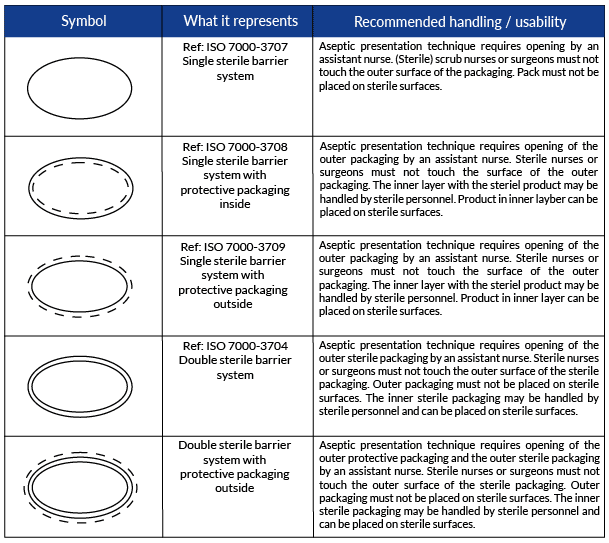
The Sterile Barrier Association recently created and validated new symbols for Sterile Barrier System (SBS) configurations for sterile medical devices.
The symbols are included in ISO 15223-1:2020, a standardized document that identifies requirements for symbols used in medical device labeling.
The reasons why these sterile medical packaging symbols were added are:
- To control specific risks with aseptic presentation
- To comply with new legal requirements deriving from the EU-MDR 2017/745
- To provide additional user benefits
Ultimately, the purpose of these new symbols is to make sure the device is safe, and effective, to use!
First off, let us explain why the addition of these symbols is necessary.
Sterile barrier systems are made up of at least one sterile barrier that allows for aseptic presentation. Sometimes, Protective Packaging (PP) is added to physically protect the SBS and the device until it’s point of use. Protective packaging can be either inside or outside the sterile barrier.
Most of the time it is easy to differentiate between the sterile barrier and protective packaging for a medical device, especially if the protective packaging is a corrugated outer box.
However, there are situations when it can be difficult to differentiate between the SBS and the PP. This confusion could alter the ability of the end-user to aseptically present the sterile contents of the packaging. Therefore, the addition of these symbols is to help the end-user understand which layer is sterile and which is not, so they are able to correctly aseptically present the device.
See the new symbols and their recommended use in the table below!
Symbol Placement
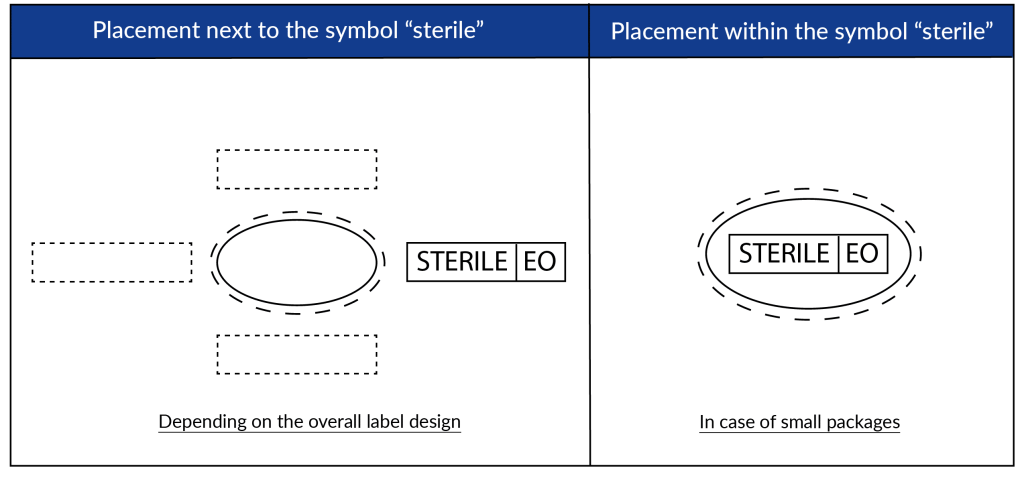
According to ISO DIS 15223-1:2020, the symbols will be printed on the medical device label adjacent to or in combination with the ‘sterile’ symbol. See below for possible configurations:
Which layers of packaging should be labeled?
While there is not a solid answer for this, we are able to make an educated assumption about which layers of packaging should be labeled by reading the MDR and ISO 11607.
MDR Requirements
The MDR requires the packaging layer that holds the sterile device has an indication on the label that it is sterile packaging. The MDR requires in annex – GSPR 11.1, that the design allows for easy and safe handling and […] prevent microbial contamination to eliminate, or reduce as far as possible, the risk of infection to the patient.
In the event of a packaging system using an SBS and PP, the MDR does not specify which layer of the sterile barrier system needs to be labeled.
ISO 11607 Requirements
ISO 11607 requires labeling for the SBS only. ISO 11607 states in subclause 6.1.8 “If the packaging system to be opened at the point of use consists of more than one packaging layer, the sterile barrier system(s) shall have an indication to be recognized as such.”
Something to keep in mind is the objective of the most recent requirement of ISO 11607:2019, usability evaluation for aseptic presentation, is to demonstrate that the design including the labels allows for easy and safe execution of aseptic presentation.
The Sterile Barrier Association guidance document states “the decision to label protective packaging should be an outcome of the risk evaluation, and packaging system design process in order to achieve acceptable usability for aseptic presentation.” For example, if the PP looks like the SBS then the validated symbols would be used to help the end-user understand the packaging system and ultimately control the risk of unintentional contamination.
Have questions about ISO 15223-1 symbols or the ISO 15223-1 update and how they pertain to your packages label requirements? Contact us today and one of our sales representatives will respond to you shortly!