
Usability Evaluation for Aseptic Presentation
ISO 11607 Part 1 requires that sterile barrier packaging systems be subject to a formal usability evaluation with real-world end-users to verify human factors considerations. When healthcare professionals can open and dispense products aseptically, it reduces the potential for accidental contamination that could impact patient health and reduces the risk for damage or loss of product.
Who is Usability Evaluation for?
Medical device manufacturers in the development process of a new product that requires sterile packaging.
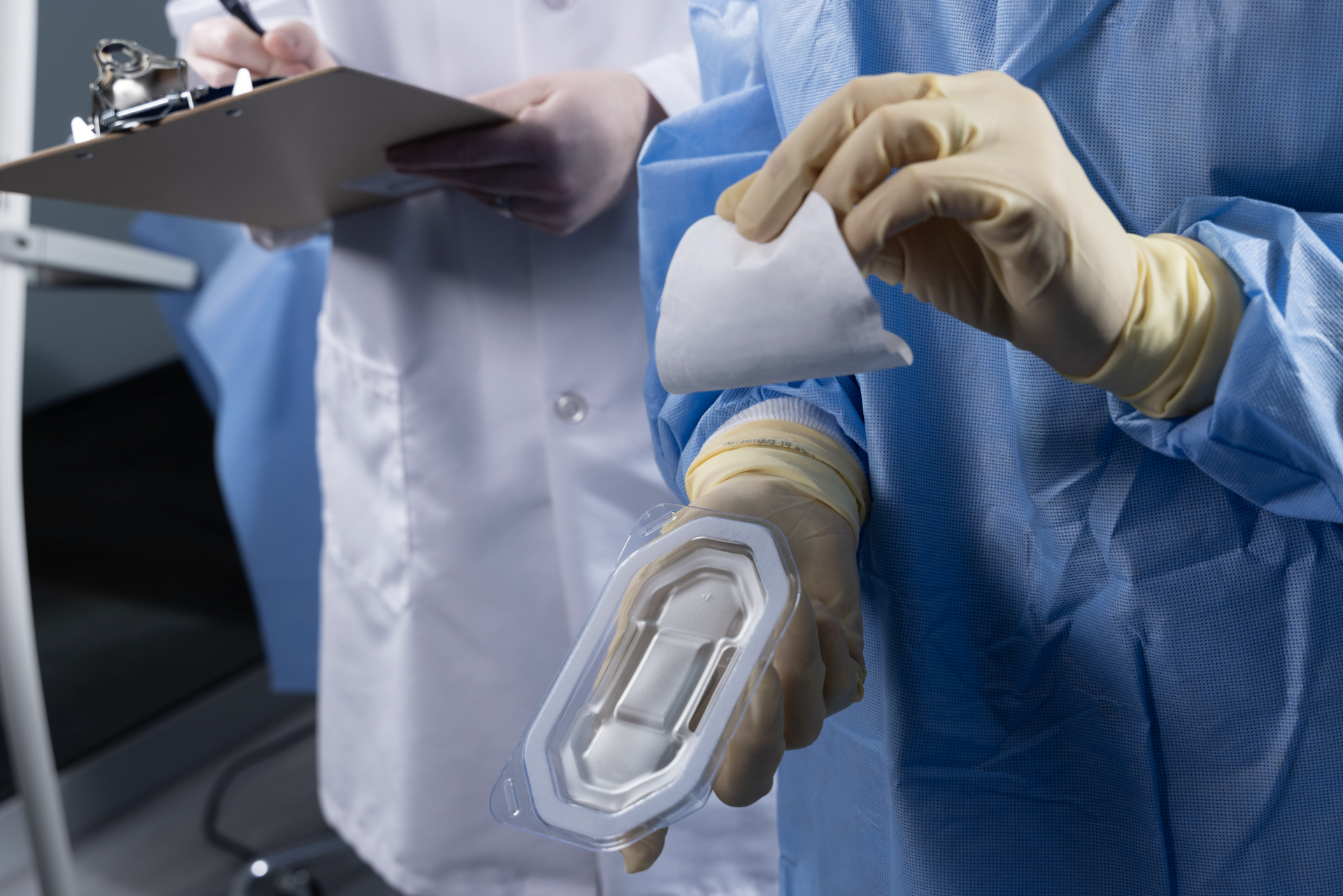
Usability Evaluation criteria
We provide Clinical Usability Evaluations to assess the three criteria for usability evaluation listed in ISO 11607:
- The ability to identify where to begin opening,
- The ability to recognize and perform the technique required to open the sterile barrier system without contaminating or damaging the contents, and
- The ability to subsequently present the contents aseptically.
All Usability Evaluations are performed in our simulated surgical suite with real-world clinicians.
Usability Evaluation Options


Let's Start Your Usability Evaluation
To get started on your usability evaluation, please provide your contact information and select an option that fits your needs or select 'I Need Guidance' if you require some assistance.
Frequently Asked Questions
Why are 15 users required for a usability evaluation?
The 15 users requirements are directly tied to requirements of IEC 62366 and AMMI/ANSI HE75.
How many samples do I need for a usability evaluation?
1 to 3 samples are suggested per user. Having a sample size on the higher end is preferred.
Can I use simulated product for my usability evaluation?
Yes, but the simulated product/device must be size and weight representative.
Can I combine my product and packaging usability sessions together?
Unfortunately, no. The Intended Users for a device are different than the Intended Users who open and dispense the product/device.
When do I perform a usability evaluation?
Formative studies should be performed before a design freeze. Summative studies should be conducted after sealing validation.